Month: June 2021
The SHED Protocol Is Online!
The SHED Protocol is Online
Study Protocols
Study protocols are a core component of a research studies. They document a study’s rationale, methodology, and planned analyses, and adherence to a protocol is now a part of research legislature (1). These have not always been publicly available, and inconsistencies between protocol and publication have been reported with selective presentation of outcomes (2), inconsistent reporting on methodology (3), and adverse events (4). Trial registries provide a platform for detailing the core components of a study (SHED’s can be found here), but protocols provide additional information beyond what is commonly available on most trial registries (5).
Protocol Publishing
As a result, there has been a move towards publication of study protocols, with entire journals now devoted to the publication of study protocols. Making protocols publicly available informs researchers and participants of upcoming trials and novel research methods, provides greater transparency, and reduces selective publication and reporting of research outcomes, although there is always work to be done (6).
However, there is a problem. Publishing protocols costs money. So how do you adhere to best research practice when you can’t afford it?
As far back as 1999 the internet has been seen as providing a solution to these problems (7). As part of a trend towards greater transparency, the use of preprint publications has grown rapidly over the last twenty years (this wonderful graph tracks the rapid growth in preprints over time). Pre-print publications are presented prior to peer review, with the caveat that it has not been peer-reviewed. During the COVID-19 pandemic the use of preprints increased exponentially as a means of rapidly reporting findings related to this new and novel disease. This image below shows the dramatic rise of preprints.
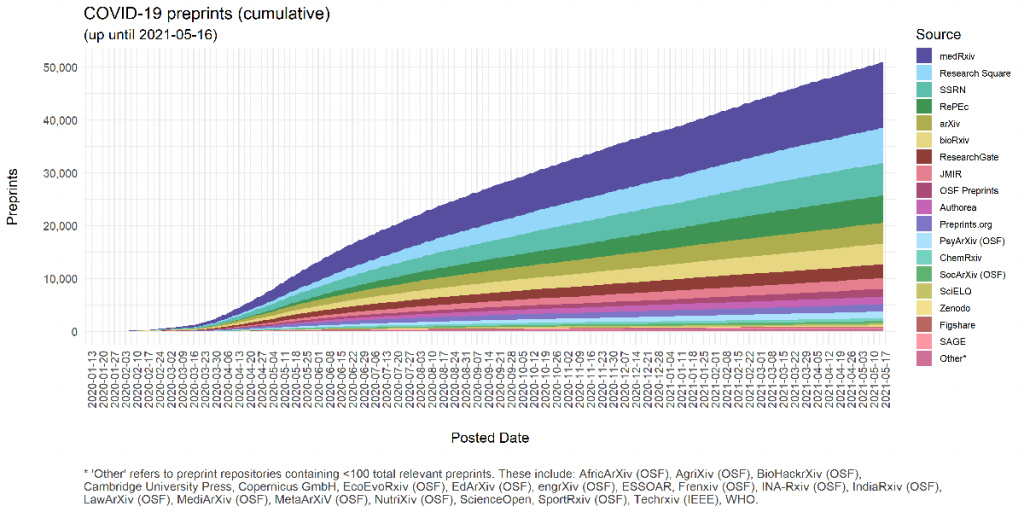
Image courtesy of Nicholas Fraser
Subarachnoid Haemorrhage in the Emergency Department (SHED)
We are choosing to publish the SHED study protocol as a preprint.
Pre-prints are normally a step towards full publication, a tool to speed up the availability of important research data. However, we are not planning on submitting SHED to a peer-reviewed journal for publication.
We believe that publishing the study protocol is vital for research transparency. Importantly, it will allow our members know more about the study, how it will be run, and get people excited for SHED. It will allow free and open-access to our methodology and ensure that we are held up to the highest research rigor without having to pay for the privilege. We want everyone to be able to read the SHED Protocol.
Despite not submitting for journal peer-review, our study has been reviewed during both the funding and ethical approval process. Indeed, many journals would take these two reviews and accept the protocol for publication – after the open-access payment of course.
Research funding is not infinite, and we have a duty to patients and those sponsoring this study to use the money wisely. We would rather use this limited resource to run the study and increase local research engagement than pay to publish the protocol on a particular journal website rather than a preprint server. Whilst many large studies will not suffer from the thorny dilemma of where best to allocate a limited research budget, many do not have this luxury.
We’re looking forward to running SHED with you all, and getting down to some non-COVID related research. Recruitment will be starting in September / October 2021. Email us here with your questions / comments / suggestions. Tweet about the study and tag us @ternfellow.
References
- European Medicines Agency. International Council for Harmonisation (ICH) E6 – Good Clinical Practice (GCP) (R2) addendum [Internet]. 2016 [cited 2021 Jun 1]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf
- Al-Marzouki S, Roberts I, Evans S, Marshall T. Selective reporting in clinical trials: analysis of trial protocols accepted by The Lancet. The Lancet. 2008 Jul;372(9634):201.
- Chan A-W, Hrobjartsson A, Jorgensen KJ, Gotzsche PC, Altman DG. Discrepancies in sample size calculations and data analyses reported in randomised trials: comparison of publications with protocols. BMJ. 2008 Dec 4;337(dec04 1):a2299–a2299.
- Scharf O, Colevas AD. Adverse Event Reporting in Publications Compared With Sponsor Database for Cancer Clinical Trials. JCO. 2006 Aug 20;24(24):3933–8.
- Skogvoll E, Kramer-Johansen J. Publication of clinical trial protocols – what can we learn? Scand J Trauma Resusc Emerg Med. 2013 Dec;21(1):12, 1757-7241-21–12.
- Cro S, Forbes G, Johnson NA, Kahan BC. Evidence of unexplained discrepancies between planned and conducted statistical analyses: a review of randomised trials. BMC Med. 2020 Dec;18(1):137.
- Chalmers I, Altman DG. How can medical journals help prevent poor medical research? Some opportunities presented by electronic publishing. Lancet. 1999;353(9151):490–3.
