update
The SHED Protocol Is Online!
The SHED Protocol is Online
Study Protocols
Study protocols are a core component of a research studies. They document a study’s rationale, methodology, and planned analyses, and adherence to a protocol is now a part of research legislature (1). These have not always been publicly available, and inconsistencies between protocol and publication have been reported with selective presentation of outcomes (2), inconsistent reporting on methodology (3), and adverse events (4). Trial registries provide a platform for detailing the core components of a study (SHED’s can be found here), but protocols provide additional information beyond what is commonly available on most trial registries (5).
Protocol Publishing
As a result, there has been a move towards publication of study protocols, with entire journals now devoted to the publication of study protocols. Making protocols publicly available informs researchers and participants of upcoming trials and novel research methods, provides greater transparency, and reduces selective publication and reporting of research outcomes, although there is always work to be done (6).
However, there is a problem. Publishing protocols costs money. So how do you adhere to best research practice when you can’t afford it?
As far back as 1999 the internet has been seen as providing a solution to these problems (7). As part of a trend towards greater transparency, the use of preprint publications has grown rapidly over the last twenty years (this wonderful graph tracks the rapid growth in preprints over time). Pre-print publications are presented prior to peer review, with the caveat that it has not been peer-reviewed. During the COVID-19 pandemic the use of preprints increased exponentially as a means of rapidly reporting findings related to this new and novel disease. This image below shows the dramatic rise of preprints.
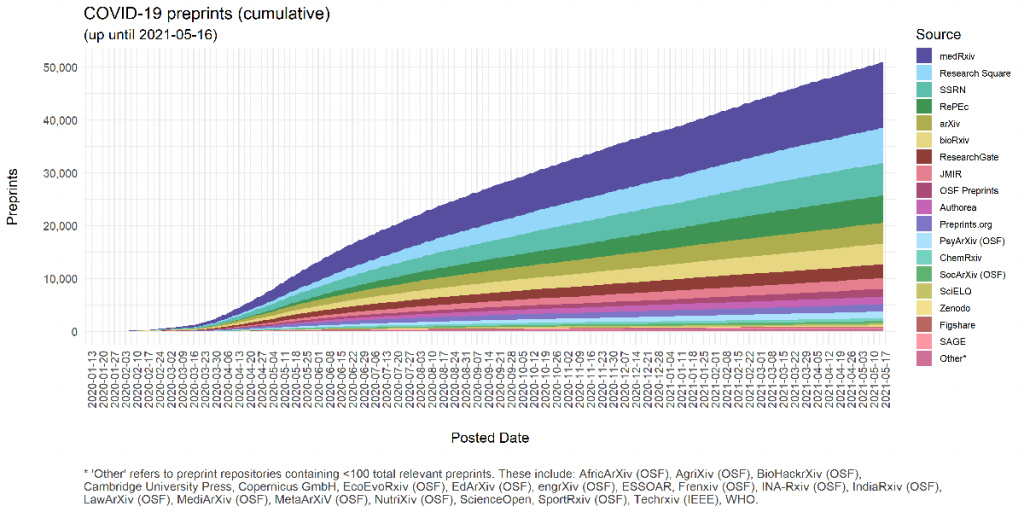
Image courtesy of Nicholas Fraser
Subarachnoid Haemorrhage in the Emergency Department (SHED)
We are choosing to publish the SHED study protocol as a preprint.
Pre-prints are normally a step towards full publication, a tool to speed up the availability of important research data. However, we are not planning on submitting SHED to a peer-reviewed journal for publication.
We believe that publishing the study protocol is vital for research transparency. Importantly, it will allow our members know more about the study, how it will be run, and get people excited for SHED. It will allow free and open-access to our methodology and ensure that we are held up to the highest research rigor without having to pay for the privilege. We want everyone to be able to read the SHED Protocol.
Despite not submitting for journal peer-review, our study has been reviewed during both the funding and ethical approval process. Indeed, many journals would take these two reviews and accept the protocol for publication – after the open-access payment of course.
Research funding is not infinite, and we have a duty to patients and those sponsoring this study to use the money wisely. We would rather use this limited resource to run the study and increase local research engagement than pay to publish the protocol on a particular journal website rather than a preprint server. Whilst many large studies will not suffer from the thorny dilemma of where best to allocate a limited research budget, many do not have this luxury.
We’re looking forward to running SHED with you all, and getting down to some non-COVID related research. Recruitment will be starting in September / October 2021. Email us here with your questions / comments / suggestions. Tweet about the study and tag us @ternfellow.
References
- European Medicines Agency. International Council for Harmonisation (ICH) E6 – Good Clinical Practice (GCP) (R2) addendum [Internet]. 2016 [cited 2021 Jun 1]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf
- Al-Marzouki S, Roberts I, Evans S, Marshall T. Selective reporting in clinical trials: analysis of trial protocols accepted by The Lancet. The Lancet. 2008 Jul;372(9634):201.
- Chan A-W, Hrobjartsson A, Jorgensen KJ, Gotzsche PC, Altman DG. Discrepancies in sample size calculations and data analyses reported in randomised trials: comparison of publications with protocols. BMJ. 2008 Dec 4;337(dec04 1):a2299–a2299.
- Scharf O, Colevas AD. Adverse Event Reporting in Publications Compared With Sponsor Database for Cancer Clinical Trials. JCO. 2006 Aug 20;24(24):3933–8.
- Skogvoll E, Kramer-Johansen J. Publication of clinical trial protocols – what can we learn? Scand J Trauma Resusc Emerg Med. 2013 Dec;21(1):12, 1757-7241-21–12.
- Cro S, Forbes G, Johnson NA, Kahan BC. Evidence of unexplained discrepancies between planned and conducted statistical analyses: a review of randomised trials. BMC Med. 2020 Dec;18(1):137.
- Chalmers I, Altman DG. How can medical journals help prevent poor medical research? Some opportunities presented by electronic publishing. Lancet. 1999;353(9151):490–3.
CERA 5
CERA 5 is here!
Good evening everyone. The COVID-19 Emergency Responsiveness Assessment study is in its fifth phase. The first three phases looking at the acceleration, peak, and deceleration of the first wave of the COVID-19 pandemic in the UK in 2020. The fourth phase (for which we now have results!) looked at the impact of the third wave. A finding observed in the deceleration phase of the pandemic was a high degree of persisting distress & trauma. Given the impact of the third wave of the pandemic, we are looking to find out how people are feeling, three months after the first survey.
If you completed the first survey, you will have received an invite to this phase of the survey. This phase of the survey - CERA 5 - is open for two weeks. We will then collect the data and send it off to the R-factory. This will allow us to assess the impact of this third wave of the pandemic in the UK, the impact of which persists for many. Our heart goes out to all those affected by the scenes of devastation seen in India.
Hope you're staying safe.
Rob
A SHED Update
SHED Update here.
Good morning everyone,
Many thanks, again, for all of the time and effort you have put into the Subarachnoid Haemorrhage in the Emergency Department (SHED) study. We have been delighted to see so much enthusiasm and engagement for this study, especially after delays due to the first peak of the pandemic.
However, this new viral variant and resultant third wave of COVID-19 is a further challenge to observational research. A new national lockdown has been enacted. Patients from Kent are being transferred to intensive care units in Devon for capacity. The urgent public health research prioritisation template has been recirculated and it is clear that any level 3 studies will receive little or no R&D support regarding set up, governance, oversight and recruitment.
As a consequence, we have discussed again with the sponsor to review delivery of the SHED study. Unfortunately, we all feel it will be difficult to proceed; the majority of sponsor R&D staff are working from home and are being prioritised to support urgent public health COVID-19 research. Your local R&D departments are likely facing similar challenges. Redeployment of nursing & clinical staff is happening, which will affect both research nurse support and clinical delivery of the study. It appears untenable to proceed at present and we remain concerned about the validity of the research within the current NHS pandemic setting.
We are therefore proposing a further deferral for SHED. We plan to defer until September 2021 for the earlier sites and October 2021 for the majority of sites.
We share your disappointment and can only apologise if rotational placement means you will now struggle to participate. However, we now have a 6-month opportunity to develop research opportunities at further sites, make the necessary amendments and ensure the protocol is deliverable in the context of any recent local changes. We have over 110 sites signed up, so chances are the ED you move to will already be involved. If it isn’t, let us know, and we can work with you to get local approvals set-up so you can hit the ground running.
It is a very difficult decision to have to do this, especially a second time, but we are certain this is the right thing to do. In addition to the above, we will be using the next 6 months to work on our website and consolidate & publish the outputs from recent TERN projects. We will also press ahead with the recently commenced Delphi project, as discussed at the December EMTA conference. You'll receive a SHED update confirming details regarding SHED nearer the time, and we'll work with you throughout.
The infrastructure and funding behind TERN provided by RCEM means that background work can continue and our administrative team can take the hit on paperwork, rather than this falling on the shoulders of enthusiastic individual researchers. This remains a big step forward for EM network research and should help us to deliver on the ideas you put forward, even if there are future hurdles to navigate.
For now, good luck, stay safe and get in touch if you have any queries.
Robert Hirst & Dan Horner
